Decaf coffee gets a bad rap, especially in the specialty coffee industry; it’s been accused of tasting bad, that the processes are unhealthy, and so on. So, we thought we’d go over the different decaffeination processes to clear up some common misconceptions, and by the end, *maybe* change your mind about it.
Decaffeination occurs at the green bean stage and is most often done by the green bean importer, after processing but before roasting. According to the Government of Canada’s Food and Drug Regulations, in order for coffee to be considered “decaf,” the bean cannot be more than 0.1% caffeine. For reference, a regular caffeinated bean’s weight is 1-2% caffeine. So, while decaf coffee is not completely caffeine-free, it contains such a small amount of caffeine that it is negligible.

The earliest decaffeination process was patented by Ludwig Roselius, a German coffee merchant of the early 1900s. His process involved soaking the green beans in hot water to open the pores, then adding a solvent called benzene that would bind to the caffeine and draw it out, leaving behind a decaffeinated green bean. Benzene is no longer used to decaffeinate green beans since it’s now recognized as a carcinogen, but the principles of Roselius’ process are still used in certain decaffeination processes today.
Decaffeination Processes
There are 3 main processes used today:
1. Chemical Process
This process relies on chemicals—the two main ones being methylene chloride and ethyl acetate—that bond to caffeine compounds to draw it out of the bean. First, the green bean is soaked in hot water or steamed, and then rinsed with either methylene chloride or ethyl acetate, which bond selectively with the caffeine compound. The beans go through a number of these chemical washes to extract as much caffeine as possible, then steamed again to remove any remaining chemicals from the bean. Finally, the bean is dried again, ready to be roasted.

Methylene chloride is a chemical used in a variety of products, including paint stripper. There have been concerns regarding the consumption of such a chemical; however, very little of it actually ends up in the final product. Most remaining methylene chloride evaporates during roasting, and any of the chemical that manages to survive is not harmful in that quantity. The alternative to methylene chloride is ethyl acetate, which is a naturally occurring chemical compound. In ripe fruit, it’s the chemical that produces the sweet smell, and it’s also a by-product of sugarcane processing. Some coffee producers in Colombia, where sugarcane is processed, are able to decaffeinate their beans themselves. More often than not, though, a synthetic version of ethyl acetate is used since it’s less expensive. Coffees that are decaffeinated with ethyl acetate are often referred to as the “Sugarcane Process” or “EA Process.”
2. Water Process
The water process relies solely on water to extract caffeine from green beans. To begin, the beans are soaked in hot water to extract all soluble materials—flavour compounds included. These beans are then discarded. The water with the bean solubles is then filtered (usually with a carbon filter or activated charcoal) to separate caffeine compounds from everything else in the water. Then, a new batch of caffeinated green beans are immersed in the water without caffeine. The caffeine in the beans diffuses into the water while keeping its own flavour compounds since the water already contains an equal amount of flavour compounds. The beans are then dried and ready for roasting.

On bags, the water process will often be referred to as “Swiss Water Process,” which is actually a patented name, specifically done by the green bean importer Swiss Water. Other companies that do water processing will likely just call the process a “Water Process.”
3. Supercritical CO2 Method
This decaffeination process uses carbon dioxide in place of chemicals or water. At a specific temperature and pressure, called the critical point, CO2 becomes a liquid. Above its critical point, the CO2 molecule is no longer strictly a liquid or a gas but has properties of both—called a “supercritical fluid” (i.e. sCO2)—and can act as a solvent.
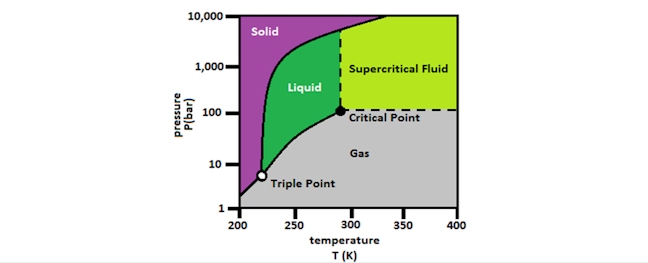
Once again, this process begins by soaking the green beans in hot water to open its pores. Then the beans are placed in a stainless-steel container, which is then sealed, and sCO2 is introduced into the vessel. The sCO2 draws caffeine out of the bean, leaving behind everything else, and then scrubbed of caffeine so it can then be recirculated to extract more caffeine from the green beans. At the end of the process, the sCO2 is moved to another vessel where the pressure of the container is released, and the sCO2 returns to its gaseous state, to be re-used.
Conclusion
Decaffeination processes have come a long way over the last 100 years. Thanks to more recent and exact processes, decaf beans arguably don’t taste worse simply by virtue of being decaffeinated. There are certainly advantages and disadvantages to each kind of process, though. For example, the chemical EA process has a lower environmental impact than the water process, but some say ethyl acetate does affect the flavour of the bean. The water process is gentler and preserves many of the original flavours and aromas but has a higher environmental impact and is more costly. The supercritical CO2 process is quite rare since it requires incredibly specific equipment and set-up, and is very expensive, but since it’s highly selective in the compounds it extracts, much of the original flavour is maintained. So, once more, and as we at Doorstep Barista always say, all that matters is your own preference when it comes to your coffee.







